Evaluating the Impact of the National Liver Review Board on Regional Variation in Quality of Transplanted Livers for Patients with and without Hepatocellular Carcinoma
Rafal D. Mazur1, David Cron2, Heidi Yeh2, David C. Chang2, Leigh Anne Dageforde2
1Harvard Medical School, Boston, Massachusetts, United States, 2Division of Transplantation; Department of Surgery, Massachusetts General Hospital, Boston, Massachusetts, United States
Objective
Patients with hepatocellular carcinoma (HCC) have historically been overprioritized in the deceased donor liver allocation system. In May 2019, the National Liver Review Board was introduced to improve equity in access to liver transplant across diagnoses by limiting HCC exception points to median model for end-stage liver disease (MELD) minus three (MMaT-3). We evaluated whether this policy change has led to regional variation in the quality of donor livers, hypothesizing that marginal quality livers would be used with higher frequency in all OPTN/UNOS regions after the policy change.
Design
Retrospective cohort study.
Setting and Participants:
We used the national transplant registry to identify all adult deceased donor liver transplant recipients from 5/18/2017-5/18/2019 (pre-policy) to 5/19/2019 - 3/1/2021 (post-policy). Patients were classified as HCC if they received allocation exception points for this diagnosis.
Main Outcome Measure
Our exposure variable was the policy change itself, and thus we compared the post- to pre-policy period. The main outcome measure was marginal quality liver. Transplanted livers were deemed marginal if they met ≥1 of the following: 1) Donation after circulatory death (DCD); 2) Donor age ≥70; 3) Macrosteatosis ≥30%; 4) Donor Risk Index (DRI) ≥ 95th percentile. For each OPTN/UNOS region, we compared characteristics across policy periods and by HCC vs. non-HCC.
Results
23,164 patients were included (11,339 pre-policy, 11,825 post-policy), 22.7% of transplanted patients received HCC exception points (pre-vs. post-policy: 26.1% vs. 19.4%). Nationally, the utilization of marginal quality livers was lower for non-HCC patients after the policy was introduced (14.1% vs. 13.0%, p=0.03), but unchanged for HCC patients (15.0% vs. 15.3%, p=0.76). We then applied the same analysis to all OPTN/UNOS regions (Figure 1, unadjusted). After adjusting for key recipient characteristics, we found no differences in donor liver quality between HCC and non-HCC in ten out of eleven regions, except for region 6, where non-HCC recipients were found to have had an increased likelihood to receive a marginal quality liver post-policy (OR: 4.5, CI:1.7 - 12.3, p<0.01).
Conclusion
While the MMaT-3 aimed to deprioritize access to LT for HCC candidates, our data show that both nationally and regionally, the policy change did not compromise donor liver quality for patients with HCC.
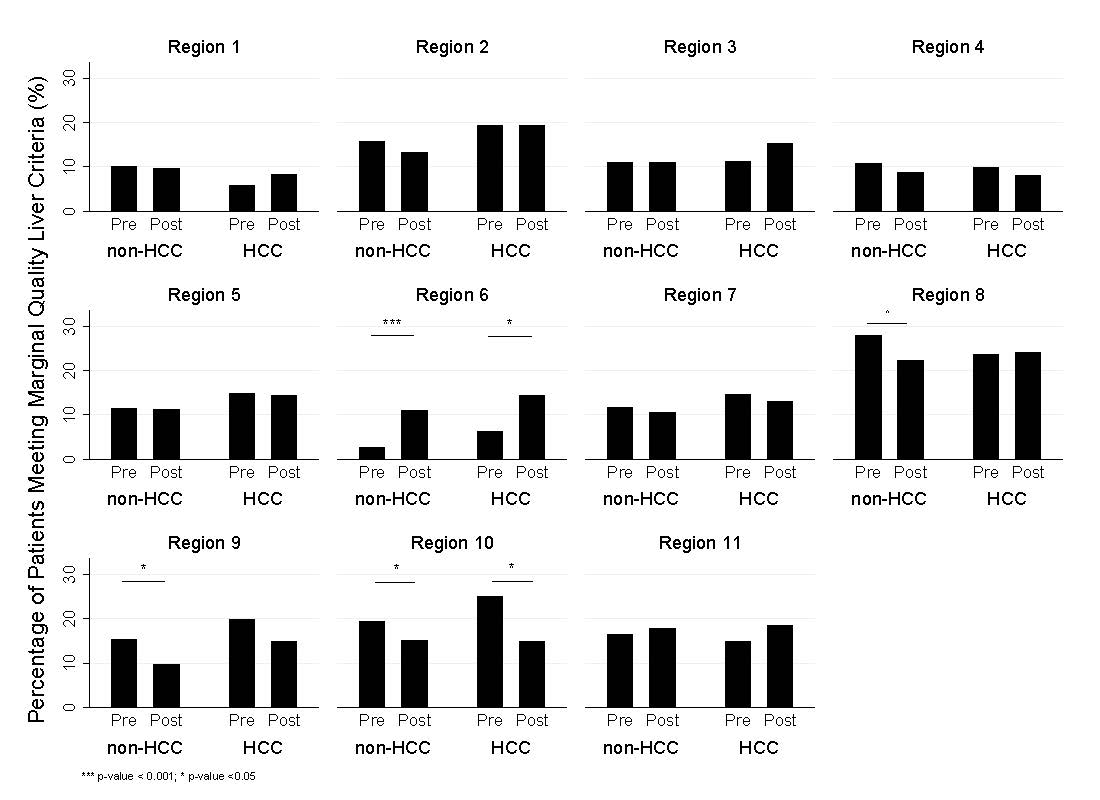
Comparison of the percentage of donors meeting the criteria for marginal quality liver transplanted into HCC and non-HCC recipients in pre- and post-NLRB periods by OPTN/UNOS region.
Back to 2022 Abstracts
