Tolerability, Attrition Rates, and Survival Outcomes of Neoadjuvant FOLFIRINOX for Non-Metastatic Pancreatic Adenocarcinoma
Zhi Ven Fong1, Carlos Fernandez-del Castillo1, Cristina R. Ferrone1, Jill N. Allen2, Lawrence Blaszkowsky2, Jeffrey W. Clark2, Ryan B. Corcoran2, Theodore S. Hong3, Aparna R. Parikh2, David P. Ryan2, Colin D. Weekes2, Jennifer Y. Wo3, Keith D. Lillemoe1, Motaz Qadan1
1Surgery, Massachusetts General Hospital, Boston, Massachusetts, United States, 2Medical Oncology, Massachusetts General Hospital, Boston, Massachusetts, United States, 3Radiation Oncology, Massachusetts General Hospital, Boston, Massachusetts, United States
Design
Cohort analysis.
Setting
Tertiary referral cancer center.
Patients
Patients with non-metastatic pancreatic adenocarcinoma.
Interventions
Neoadjuvant FOLFIRINOX.
Main Outcome Measure(s)
Completion of FOLFIRINOX chemotherapy.
Results
Of 254 patients who initiated first-line neoadjuvant FOLFIRINOX, 199 (78.4%) underwent exploration. A total of 142 (55.9%) patients were resected, and 129 were R0 (50.8%). The pathologic complete response rate was 5.8%.
Among the 142 explored and resected patients, 67 (47.2%) had no progression, 14 (9.9%) progressed locally, and 61 (42.9%) developed distant metastases over a 46.3-month median follow-up period.
Fifty-four (21.3%) patients did not complete their chemotherapy cycles (median 4 of 8 cycles) due to poor tolerability (44.4%), disease progression (31.5%), and poor response (14.8%), among other causes (9.3%). A total of 216 (85.0%) patients experienced FOLFIRINOX-related toxicity with 109 (42.9%) experiencing grade 3/4 toxicity. A total of 73 (28.7%) and 99 (40.0%) patients required an ED visit or inpatient admission, respectively.
One hundred and thirty-four patients required a biliary stent, of whom 40 (29.9%) required additional ERCP intervention for obstruction/cholangitis. Importantly, however, the need for additional intervention was not associated with likelihood of completing FOLFIRINOX or undergoing surgical exploration.
Finally, inability to complete FOLFIRINOX therapy was not itself associated with an impact on overall survival (OS). However, not undergoing exploration was associated with impaired OS (12.5 months vs. 39.7 months, p<0.001). Independent predictors of not undergoing exploration were prior receipt of chemotherapy (OR 0.06, p=0.02), inability to complete FOLFIRINOX cycles (OR 0.2, p=0.003), increase in ECOG score (OR 0.2, p<0.001), and being single/divorced (OR 0.3, p=0.018).
Conclusions
This series reports on a complete denominator of all patients with non-metastatic PDAC initiated on neoadjuvant FOLFIRINOX and provides a report of tolerability, attrition rates, and toxicity. Overall, 21.6% of patients did not undergo surgical exploration. Inability to complete FOLFIRINOX cycles was not associated with worse OS, but not undergoing exploration was. Finally, marital status and declines in ECOG scores highlight the importance of optimized social support and prehabilitation in the neoadjuvant setting.
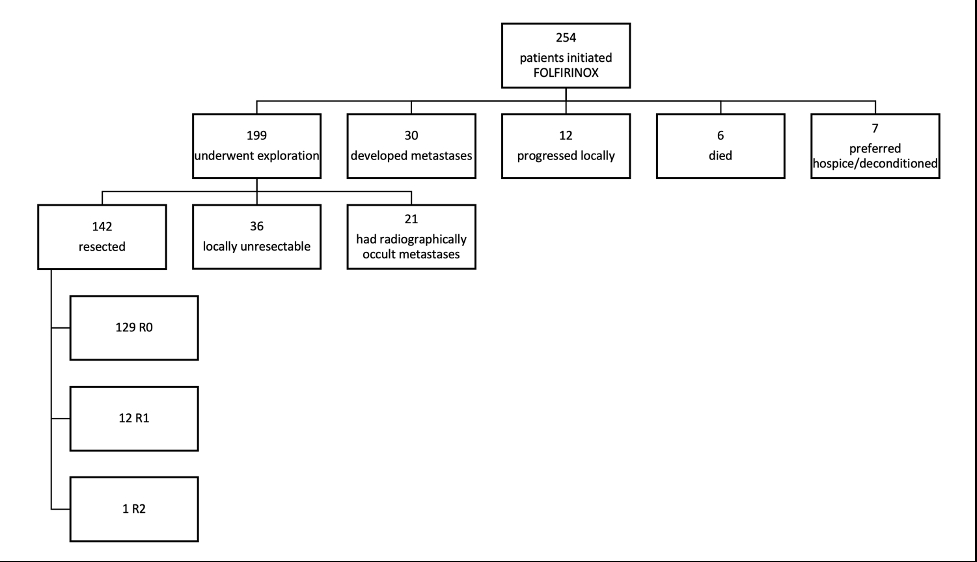
Flow diagram for patients with non-metastatic pancreatic adenocarcinoma who initiated FOLFIRINOX treatment from 2015 to 2020.
Back to 2022 Abstracts
