Individualizing Care for Gallbladder Cancer with Use of a Survival Nomogram
Laura M. Nicolais, Timothy Fitzgerald
Surgery, Maine Medical Center, Portland, Maine, United States
Objective: The rarity and lack of Level I Evidence compromise our ability to care for patients with gallbladder cancer. Existing prospective randomized trials often combine adenocarcinoma of the gallbladder with other neoplasms such as extrahepatic cholangiocarcinoma. Given this, it is difficult to understand the benefits of current standard-of-care therapy for individual patients.
Design: Retrospective cohort study utilizing NCDB data between 2004 and 2018. Survival analysis was performed stratified by stage.
Setting: Commission on Cancer accredited cancer centers in the United States.
Patients: Patients with resected Stage Groups IB-IVA gallbladder adenocarcinoma were included. Those with metastatic disease or treatment with neoadjuvant therapy were excluded.
Results: A total of 9,050 patients met inclusion criteria (Stage IB: n= 1655, Stage II: n=3824, and Stage III/IVA: n=3571). Overall median survival was 29.8 months (CI 28.6 - 31.2): Stage IB: 67.0, Stage II: 36.6, and Stage III/IVA 18.4 months. A majority of patients (65%) underwent lymph node dissection and a minority (38%) were treated with adjuvant therapy: 19% chemotherapy and 19% chemoradiotherapy. Use of adjuvant therapy increased as the stage group increased (Stage IB: 7 and 7%; Stage II: 16 and 17%; Stage III/IVA: 28 and 26%, for chemotherapy and chemoradiotherapy, respectively). A clear survival benefit was noted for additional surgery beyond simple cholecystectomy for all stages of disease. Regional lymphadenectomy was associated with improved survival for Stage IB (105 vs. 36 months, HR 0.71, p < 0.001), Stage II (56 vs. 20 months, HR 0.67, p < 0.001), and Stage III/IVA (23.8 vs. 9.9 months, HR 0.66, p < 0.001). The impact of adjuvant therapy was stage-dependent. Adjuvant chemotherapy improved survival only in Stage III/IVA (20 vs. 12.2 months, HR 0.70, p < 0.001) with no survival advantage in the lower stage groups (Stage II 42.9 vs. 33.5 months, HR 1.02, p=0.828) and a trend toward harm on multivariable analysis in Stage IB (65 vs. 68 months, HR 1.34, p= 0.079). Conversely, adjuvant chemoradiotherapy was associated with survival benefit in all stage groups: Stage IB 102 vs. 64.8 (HR 0.86, CI 0.58 - 1.90, p = 0.456), Stage II 49 vs. 33.5 months (HR 0.79, CI 0.67- 0.93, p = 0.005) and Stage III/IVA 31 vs. 12.2 (HR 0.62, CI 0.53 - 0.71, p < 0.001). A nomogram can be used clinically to apply these findings and assess the incremental survival benefit for various treatment modalities (Figure 1).
Conclusion: Clinical data can be utilized to construct a nomogram that provides guidance for the treatment of adenocarcinoma of the gallbladder. Also, additional surgery beyond simple cholecystectomy provides benefit to all patients. Although adjuvant chemotherapy is the standard of care, chemotherapy is beneficial to only patients with higher staged disease. Interestingly, chemoradiotherapy is universally beneficial.
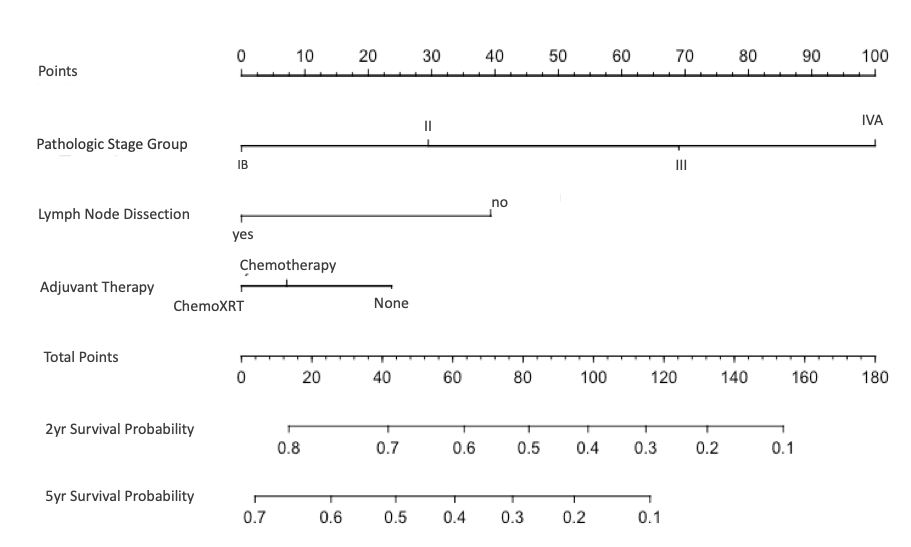
Figure 1: Nomogram to predict 2-year and 5-year survival probability.
Back to 2022 Abstracts
